The National Childhood Vaccine Injury Act of 1986 established the National Vaccine Injury Compensation Program (VICP) as a no-fault alternative to the traditional tort system. VICP provides compensation to individuals found to be injured by certain vaccines. Even without a formal finding, petitioners may still receive compensation through settlements.
The program was created in response to lawsuits against vaccine manufacturers and healthcare providers that posed a threat to vaccine availability and vaccination rates. VICP started accepting petitions in 1988.
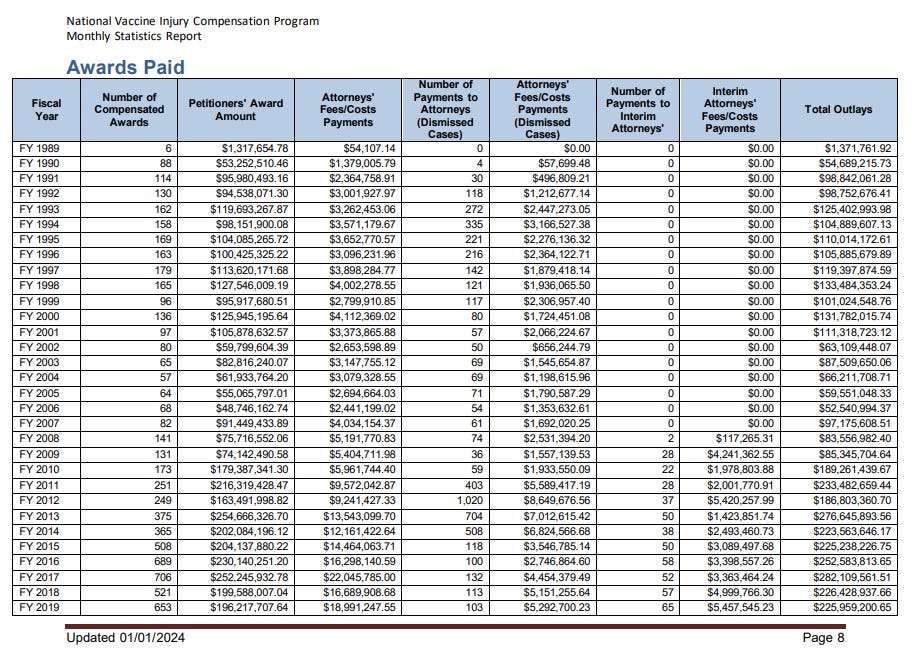
In 2023 the U.S. government paid out $173,842,001.56 to 885 people.
Click here to read the latest report released by the government on payouts in this program.
The following is a chart of all of the payouts dating back to 1989.
It’s important to note COVID vaccine injuries are not covered as part of this program. The United States does have a program to compensate for COVID vaccine injuries but they are not releasing a lot of information about payouts.
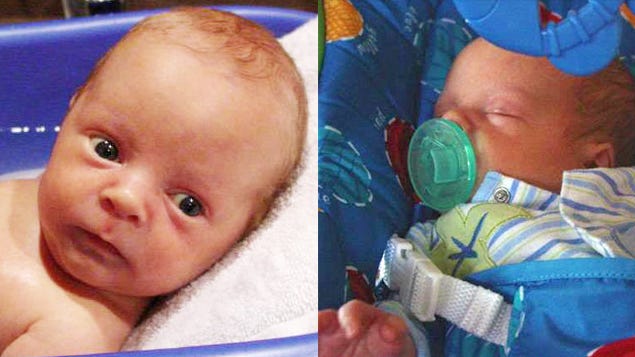
After receiving his first set of vaccines, two-month-old Alexander experienced a severe seizure leading to cardiorespiratory arrest and irreversible brain damage, resulting in his tragic death. The parents, devastated and initially denied by the doctor, embarked on a quest for answers. Despite skepticism from medical professionals, an independent autopsy, expert testimonies, and legal battles in the Vaccine Injury Court proved the link between the vaccines and the tragic outcome. Ultimately, they were awarded $250,000 in recognition of the vaccine-related injury and loss.
Learn more about Alexander’s case here.
The following are the vaccines covered in the government program that is offering payouts:
Haemophilus influenza type b polysaccharide conjugate vaccines (e.g., Hib)
Seasonal influenza (e.g., Flu)
Note: Non-Seasonal Flu Vaccines are not VICP-covered vaccines.More detailsMeningococcal (e.g., MCV4, MPSV4, MenB-FHbp, MenB-4C)
Note: All other formulations of meningococcal vaccines, such as vaccines produced by recombinant DNA technology, are covered under the VICP in otherwise eligible individuals.Pneumococcal conjugate (e.g., PCV)
Note: Pneumococcal polysaccharide vaccine (PPSV, PPV) is not a VICP-covered vaccine.
Note: Herpes zoster (shingles) vaccine is not a VICP-covered vaccine.
What vaccine liability protection is afforded to vaccine manufacturers and administrators?
The National Vaccine Injury Compensation Program (VICP) is an alternative to the tort system for resolving vaccine injury petitions. Whether a vaccine manufacturer or administrator is afforded the liability protections of the National Childhood Vaccine Injury Act of 1986, as amended, (the Act) depends upon whether the vaccine is covered under the VICP.
Under the Act, persons with petitions of vaccine-related injuries or deaths resulting from covered vaccines must first exhaust their remedies under the VICP before they can pursue legal actions against vaccine manufacturers or administrators.
To exhaust the remedies available under the VICP and pursue a legal action against a vaccine manufacturer or administrator outside of the VICP, a VICP petitioner must either withdraw his or her petition (if the special master of the U. S. Court of Federal Claims (Court) has failed to issue a decision or the Court has failed to enter judgment within the time provided by the Act) or reject the judgment under the VICP.
Although the Act provides liability protections to vaccine manufacturers and vaccine administrators who administer covered vaccines in many circumstances, these protections are not absolute.
There are instances when a vaccine manufacturer or administrator who gives a covered vaccine is not protected from liability by the Act, such as when an individual files a petition and is requesting damages of $1,000 or less. In this case, a civil suit against a vaccine manufacturer or an administrator may be permitted to be filed in state or federal court without first filing a petition in the VICP.
In addition, if the VICP has paid a petitioner for a vaccine-related injury, the VICP may be able to pursue its own action against a vaccine manufacturer or administrator using its subrogation rights.
Did any of you know this program even existed? I would not be allowed to discuss this program on Facebook or even TikTok.