DALLAS, TX - In a landmark decision reached in federal court on Thursday, the U.S. Department of Health and Human Services (HHS) and the Food and Drug Administration (FDA) have conceded to withdraw social media posts and an online article cautioning against the off-label use of ivermectin in treating COVID-19. This agreement comes after legal action initiated by Dr. Mary Talley Bowden, a Houston-based Ear, Nose, and Throat specialist, alongside two other physicians in 2022, accusing the FDA of impeding their patient care efforts.
"After nearly two years and a resounding rebuke by the Fifth Circuit Court of Appeals, the FDA has agreed to remove its misleading social media posts and consumer directives regarding ivermectin and COVID-19," stated Bowden in a communication to The Texan.
I walked away from the mainstream media to launch my independent journalism. Please help support my mission to get articles like this out free to everyone. Become a paid subscriber to help my mission of telling you stories the media doesn’t want you to see.
The legal battle stemmed from allegations that FDA statements were hindering doctors' ability to treat COVID-19 patients effectively. Dr. Bowden, among thousands of other physicians, had been exploring treatment options for the virus since its emergence in the United States in 2020. Despite ivermectin's FDA approval for human use since 1987, Bowden claimed that the FDA's campaign against its use led to challenges in obtaining the drug, with pharmacists refusing to dispense it and insurance companies declining coverage.
The lawsuit highlighted FDA publications and actions, including a 2021 social media post featuring a horse with a caption discouraging the use of ivermectin in humans, as well as an article titled "Why You Should Not Take Ivermectin to Treat or Prevent COVID-19." Dr. Bowden asserted that the FDA's stance against ivermectin failed to acknowledge the drug's legitimate prescription use by physicians.
Initially, the lawsuit faced hurdles when Judge Jeffrey Brown of the U.S. Southern District Court in Texas dismissed the case citing FDA's sovereign immunity. However, the Fifth Circuit overturned this ruling last September, remanding the case back to the lower court. Judge Don Willett, writing for a panel of three Fifth Circuit judges, emphasized that while the FDA can inform, it cannot dictate medical decisions.
Following subsequent legal proceedings, only Dr. Bowden was granted standing to sue the FDA. However, before the case could progress further, HHS initiated settlement discussions, leading to an agreement to dismiss the case "with prejudice." As part of the settlement terms, the FDA is required to remove the contentious article and related social media posts within 21 days.
Even to this day outright lies by the media are showing up on social media. Take a look at this fake news headline from the Inside Edition.
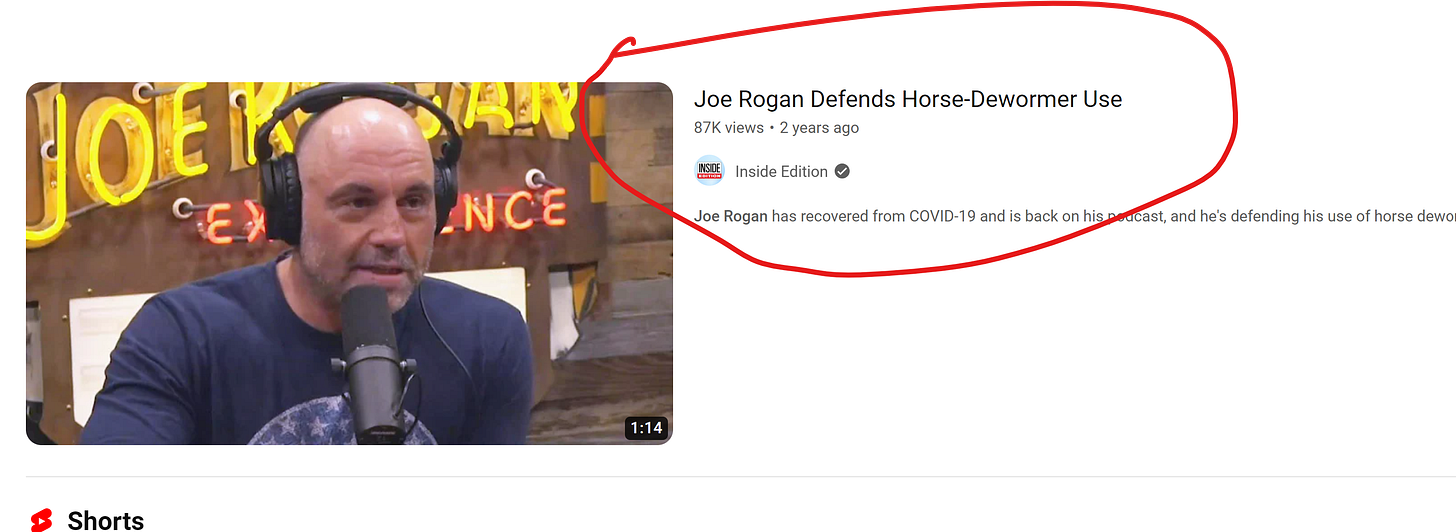
Dr. Bowden expressed relief at the resolution, stating that while the damage caused by the FDA's actions would linger, future patients would be shielded from similar governmental interference in medical care.
In a separate development, the Texas Medical Board (TMB) had filed charges against Dr. Bowden last year, alleging violations of medical standards and dissemination of disinformation. However, the case has now been moved to private mediation, delaying the public hearing scheduled for April 2024.




















